Please enjoy our news here.
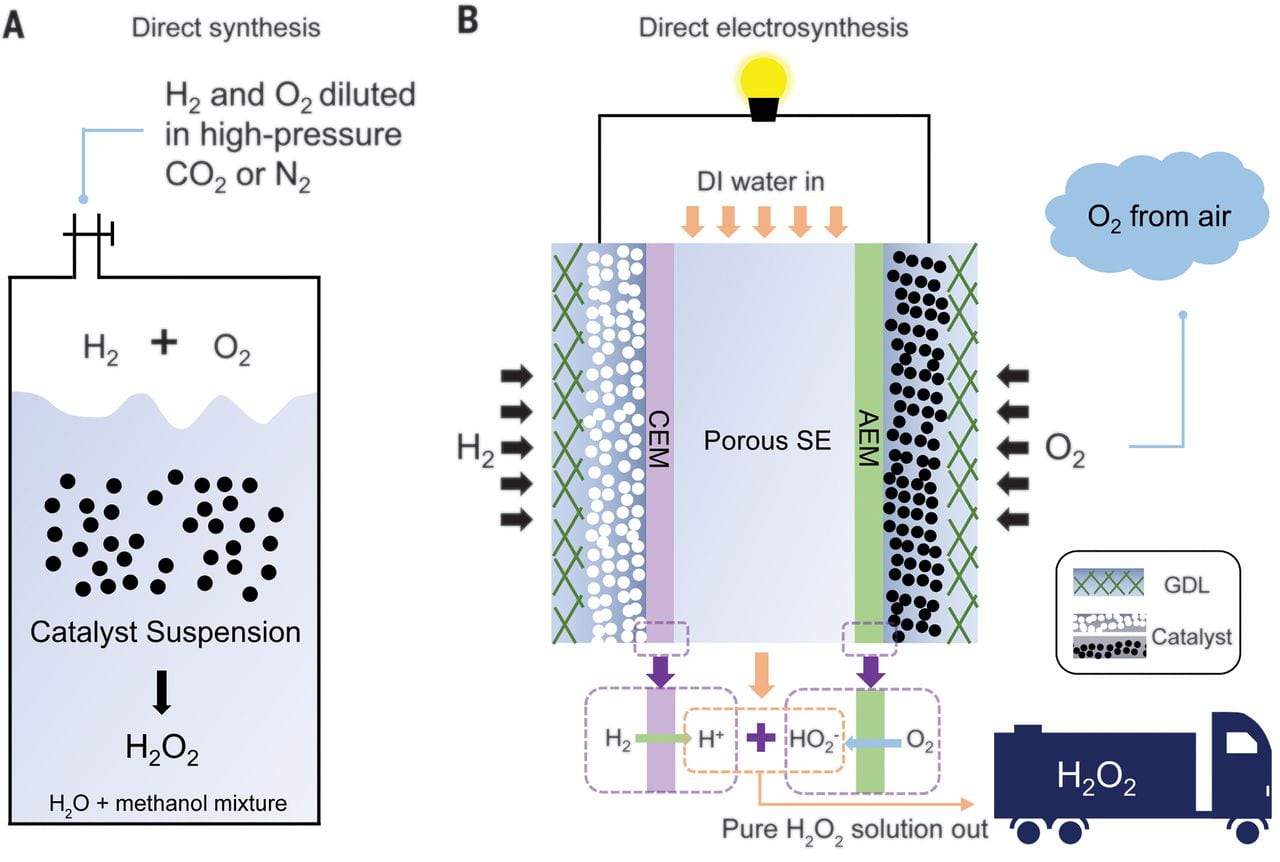
Hydrogen peroxide (H2O2) synthesis generally requires substantial postreaction purification. Here, we report a direct electrosynthesis strategy that delivers separate hydrogen (H2) and oxygen (O2) streams to an anode and cathode separated by a porous solid electrolyte, wherein the electrochemically generated H+ and HO2– recombine to form pure aqueous H2O2solutions. By optimizing a functionalized carbon black catalyst for two-electron oxygen reduction, we achieved >90% selectivity for pure H2O2 at current densities up to 200 milliamperes per square centimeter, which represents an H2O2 productivity of 3.4 millimoles per square centimeter per hour (3660 moles per kilogram of catalyst per hour). A wide range of concentrations of pure H2O2 solutions up to 20 weight % could be obtained by tuning the water flow rate through the solid electrolyte, and the catalyst retained activity and selectivity for 100 hours.

‘One small step for man, one giant leap for mankind’
Congratulation!!
Is there anyway to contact the group? I am heading a commercial agro project in Nicaragua, and we are looking at ways to produce hydrogen peroxide as it is hard to come by. I would like to find out more about the technology. Thks
This is definitely going to be industrialized!
However, after carefully reading your artical and supporting materials, I still have some questions in the oxygen reduction process:
1. What is the real resistance of the hole electrolyzer, or what are resistances of each part of the electrolyzer, especially prous SE?
2. What are the diffusion potentials between Nafion/Porous SE/AMI 7001? In what roles these potentials play in your electrolyzer?
3. What is the most important reason you think about the large current density your electrolyzer could reach?
Could you please answer questions above? I am very intersted in H2O2 electrochemical synthesis.
The series resistance of our electrolyzer with SSE is about 2-3 ohm. We do not measure the diffusion potentials between Nafion/SE/AMI 7001, which drives the diffusion of the formed protons and HO2- anions into middle SE layer. The low cell resistance and high purity of the feedstocks guaranteed the large H2O2 production rate under relative low cell voltage in our case.
This discover is very exciting! I’d like to use this to assist those without access to clean water. Are you able to send through where I can look at the catalyst etc. for me to see how I can use this for that application?
How can i get one? I’m a spirulina and mycelium farmer in need of constant h2o2. Thanks
We are currently developing a commercial product and please keep a close eye on us!
We are looking to produce 2000 ton per year. Is this commercially available yet.
About time! And earnestly sought!
With vacuum distillation to increase concentration and spurious additions, I see this chemical supplanting fossil fuels and various tricks will get us to that point where we can breathe again. Important!
Please call me at 309-840-3196 ASAP!
And Congratulations!