“Electrifying Chemical Engineering”
We appreciate great supports from funding agencies.
With the fast development of clean technologies to harvest clean electrons from the solar or the wind, we are about to entering an era with cheap, abundant, and clean electrons. This has already trigged revolutions in traditional chemical engineering industries, with alternative routes for chemicals/fuels productions as well as energy storage and usage. How to efficiently store and make use of those electrons becomes our central interests.
Schematic illustration of carbon cycle via CO2 electrolysis powered by renewable energy sources such as wind, solar, and hydro. Once the produced electrofuels are combusted they will release CO2 which can then be recycled back, effectively closing the carbon loop. Novel heterogeneous catalyst design plays a key role in the electrochemical reduction of CO2 into fuels and chemicals toward more efficient renewable energy storage and utilization.
The research in Wang Group is currently focused on exploring highly efficient catalysts for very important catalytic reactions, including carbon dioxide reduction, H2O2 generation, N2 reduction, water splitting, fuel cell electrocatalysis, and so on. Coupling electrochemical redox reactions for high-energy rechargeable batteries is also what we explore. Those fundamental reactions play critical roles in practical applications, ranging from renewable energy conversion and storage, chemical or fuels production, water treatment, agriculture fertilization, and so on. Different types of tuning methods in catalytic materials have been developed in the Wang Group, including size tuning from bulk materials to isolated single atoms, facets tuning for varied catalytic performances, coordination tuning for changing the intermediate binding strength, and so on. Our research interests range from fundamental mechanism understandings to practical applications of our technology.
1. CO2 reduction to C1/C2/C3 products
Cover arts of our recently published CO2 reduction papers. Left: single atom catalyst for CO2 reduction to CO; Right: metal ion cycled Cu foil with Cu nanocubics covering the surface.
Electrochemically reducing CO2 gas into a variety of products, ranging from CO and formic acid as C1 products, ethylene and ethanol as C2 products, n-propanol as C3 products, and so on, is one of the most important thrusts in my group. The challenge of this technology comes from the competing hydrogen evolution reaction, or water reduction. Imagining you have 100 electrons, you want even more than 99 of them can be dumped into CO2, instead of the case that 99 of them goes into H2O for H2. However, the reality is, most of the electrocatalytic materials choose the latter way. The key to solve this problem relies on precisely engineering the materials properties for selective reduction of CO2 molecules towards desired product.
The detailed video protocol for CO2 reduction testing and single atom catalyst preparation.
Over the past few years our group have developed a series of high-performance catalysts, including transition metal single atom catalysts, metal-ion cycled Cu catalyst, and Li ion tuned Zn catalyst.
Atom Probe Tomography of Ni single atom catalyst. Each of the pixel represent one single atom, which was ejected out of the sample, detected by mass spectrum, and reconstructed. Red represents C, green represents Ni, and blue represnets N. This technology becomes very important in detecting the local coordination environment of metal single atoms.
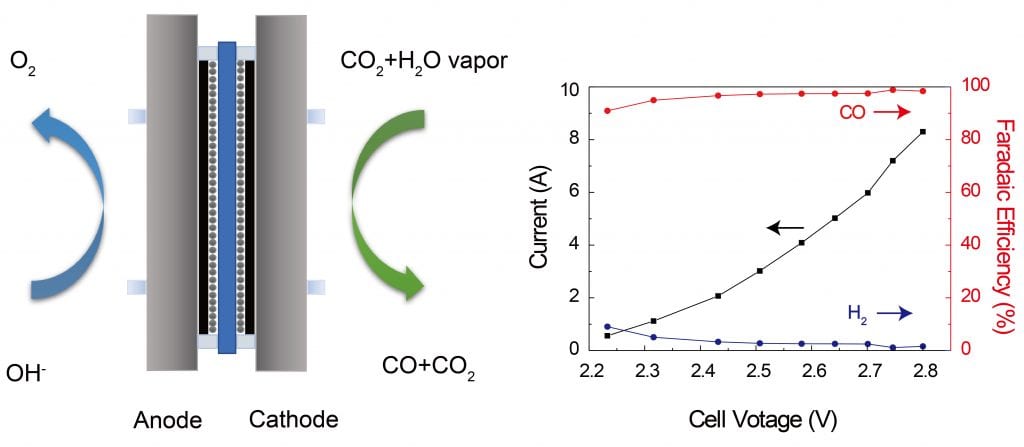
With the step-by-step development we recently demonstrated a scaled-up CO2 reduction to CO system, in terms of low-cost synthesis of single atom catalyst, large current densities (>100mA/cm2) with almost 100% CO selectivity. Moving forward our group will be focused on how to further upgrade the CO2 reduction products.
Coupling low-cost single atom catalyst with gas-phase electrochemical reactor (to separate catalyst with liquid water), we demonstrated a unit cell with 99% CO selectivity under more than 8A (3.34L/h) CO generation rate.
2. Electrochemical H2O2 generation
Molecular oxygen (O2) can be electrochemically reduced to water (H2O) via a 4e– pathway, or hydrogen peroxide (H2O2) with 2e– transferred in aqueous solutions. The former pathway is preferred in fuel cell applications to maximize the energy conversion efficiencies, and the latter one represents a green synthetic method for H2O2. Compared to the traditional energy, capital, and waste intensive anthraquinone process, electrochemical synthesis of H2O2 becomes a promising alternate with significant advantages including: (1) mild reaction conditions under room temperature and ambient pressure; (2) renewable electricity as the energy source without fossil fuel consumptions; and (3) green precursors starting with water and air. Our group is currently focused on developing both catalytic materials and electrochemical devices for the practical applications of on-site H2O2 generation.
Animation illustration of direct electrosynthesis of pure H2O2 solutions using solid electrolyte.
3. Water splitting and fuel cell catalysis
With more and more solar panels installed on family houses, as well as the fast implementation of solar or wind farms all over the world, a powerful energy storage system is urgently needed to overcome the daily and seasonal mismatch between the production and usage of clean electricity. In addition, an efficient energy storage system for “peak shaving”, which is used to reduce electrical power consumption during periods of maximum demand on the power utility, can dramatically reduce the utility costs. Hydrogen (H2) as an efficient energy carrier, which can be generated via electrocatalytic water splitting (2H2O = 2H2 + O2; E0 = 1.23 V) and converted back to electricity through fuel cell stacks, becomes a more attractive energy storage system compared to Li ion batteries especially in large-scale applications: 1) Large-scale energy conversion: Different from batteries where the three-dimensional (3D) volume of electrode materials need to be linearly scaled up to for increased energy storage, water-splitting electrolyzers only need to increase the 2D electrode surface areas to accommodate the increased capacity. The produced H2 gas can be accumulated in high-pressure tanks, dramatically reducing the energy storage cost. In short, while the storage of Li ions needs to have graphite and metal oxide materials as hosts, pure H2 gas can be stored efficiently; 2) Long-term energy storage: Excess H2 gas produced by solar panels in summer can be continuously accumulated and stored for the use during winter, circumventing the problem of self-discharging in batteries during long-term energy storage; 3) Efficient energy distribution: While batteries cannot be easily moved, clean H2 produced from concentrated solar or wind farms can be transported via pipelines to the end use in cities. More importantly, the pipeline distribution to gas stations would further facilitate the popularization of H2 fuel cell vehicles.
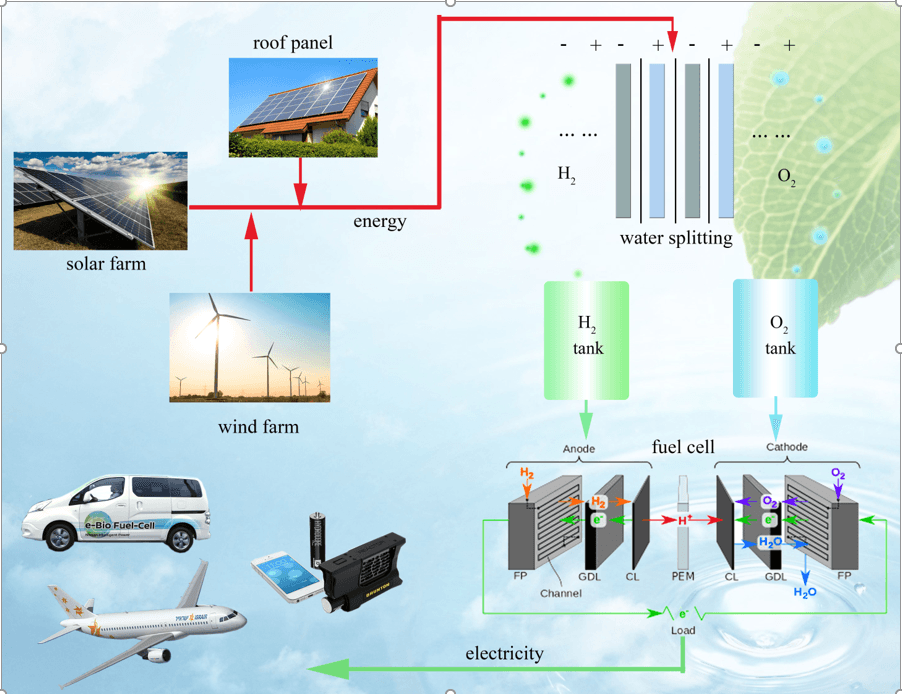
Schematic of H2 application in energy storage and usage
Tremendous efforts over the past decade have been focused on exploring alternative catalytic materials, including a variety of earth-abundant transition metal based catalysts, to replace traditional noble metals such as Pt, Ir or Ru. Different from traditional approaches in designing varied nanocatalysts, we have successfully developed a systematic “electrochemical tuning method” to controllably tune the electronic band structure of existing catalyst, and thus tune its catalytic performances within a wide range. This tuning method includes Li intercalation tuning, Li extraction tuning, Li cycling tuning, battery electrode strain tuning, and so on. One representative example is the direct and continuous strain control of Pt catalyst with tunable battery electrode materials. The Pt lattice can be controllably compressed or stretched under different battery charging/discharging status.
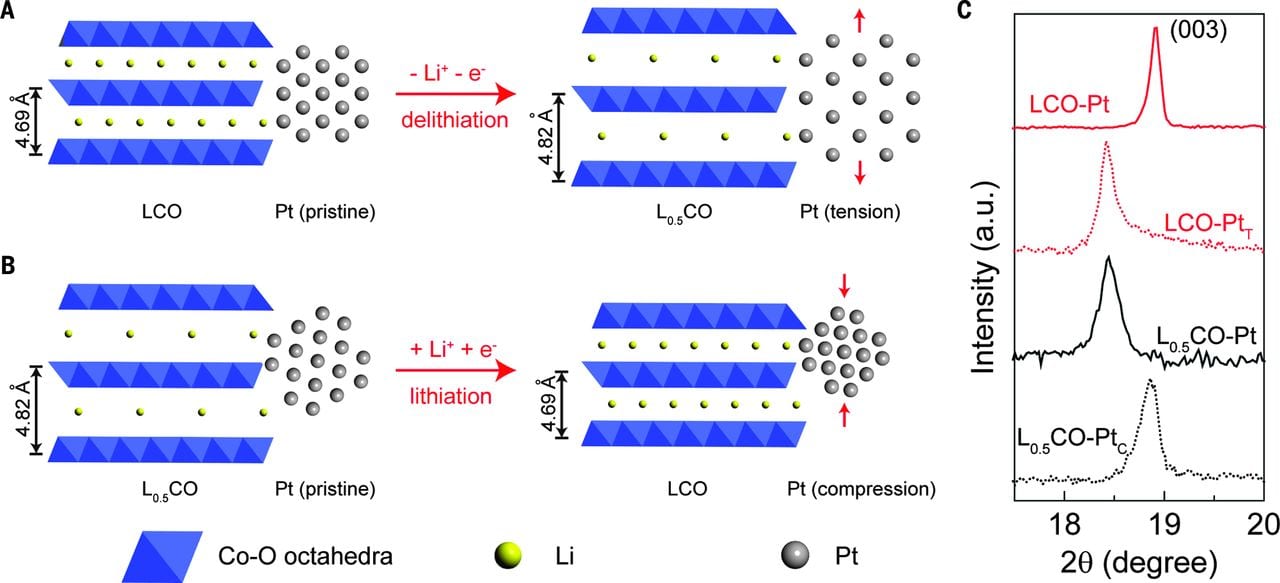
Schematic of the lattice constant change of LCO substrates and how the lattice strains are induced to Pt NPs.
Until today, most of the efforts in this field have been focused on developing catalytic materials only, but few were carried out for 1) scalable catalyst synthesis on current collectors, and 2) practical device design toward large-scale H2 generation. To build up a bridge connecting fundamental water-splitting catalysts with their real applications in industry in the future, our group started to design a modular alkaline water-splitting electrolyzer system with scaled-up metal foam electrodes covered by low-cost transition metal catalysts, which are made of Fe, Ni, and Mo, for efficient water splitting. An electrolyte circulation system facilitates the mass transport and thus can further boost the H2 generation particularly under large currents. As a very preliminary result, the overall water-splitting performance of one-unit cell with a dimension of 10×10 cm2 under room temperature presents an early onset voltage of 1.54 V (energy efficiency of 80%), and delivered practical currents of 20 and 55 A (9.1 and 25.0 L/h H2 generation) under 2.2 and 2.9 V without iR compensations, respectively. This demonstration could stimulate new focuses in water-splitting towards more practical applications. A stack with about 20 unit cells is expected to be capable of storing the electricity generated by solar panels on the roof of a single family house.
Clean H2 generation via our electrocatalytic water splitting system with a unit cell of 10×10 cm2. The H2 generation in this video is under 55A, which equals to a rate of 25L/h.
4. Electrochemical ammonia synthesis
Exciting projects are undergoing and more to be included in the future.
5. Smart window for energy-efficient buildings
Exciting projects are undergoing and more to be included in the future.